Combination therapy
No results were found for your selected species
Stromease
Active substance
ATC code
Species
Dogs and cats
Indications
Supportive treatment of corneal ulcers.
Dose to be administered and administration route
Ocular use.
The veterinary medicinal product is to be administered into the affected eye(s), at a dose of 2 eye drops, 3 to 4 times daily.
Instructions for opening the container and attachment of dropper applicator:
• Wash hands carefully in order to avoid microbiological contamination of the content in the vial.
• Flip open the metal cap and pull it all the way down along the pre-cut lines. Then remove the rest of the metal seal (picture 1).
• Remove the orange colored stopper (picture 2) from the vial.
• Do not touch the opening of the vial after removing the stopper.
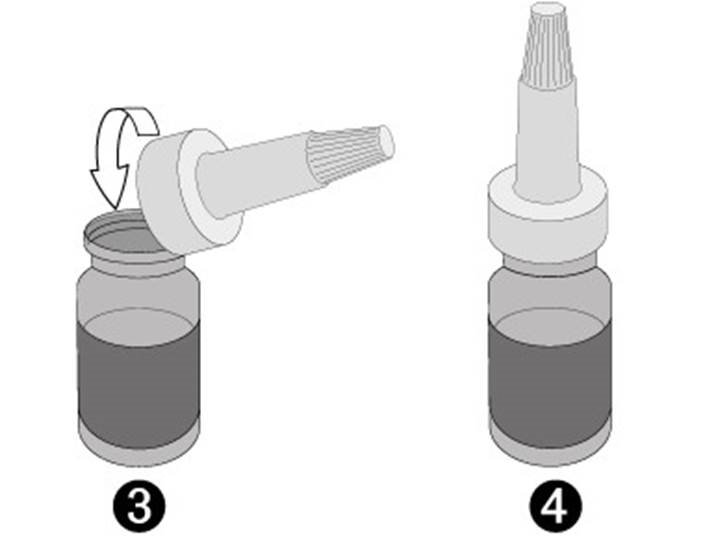
• Take the dropper with the small white screw cap on the top out of its sachet, without touching the end intended to be attached to the vial, attach it (picture 3) to the vial and do not remove it anymore.
• The veterinary medicinal product is now ready for use (picture 4).
• 
Instructions for use:
Remove the small white screw cap to administer the veterinary medicinal product. Keep the dogs/cats head steady in a slightly upright position. Hold the container in an upright position without touching the eye. Rest your hand/little finger on the forehead of the dog/cat to maintain distance between the container and the eye. Gently pull the eyelid of the affected eye downwards, this will form a little eyelid pouch. Gently squeeze the dropper to administer two drops into the eyelid pouch that you created.
Be careful not to touch the dropper tip after opening the container and replace the white cap after use. Place the container back into the carton in the upright position and store out of sight and reach of children until the next medication.
Treatment should be continued in accordance with the instructions given by the individual veterinarian.
When treatment is combined with other ocular products, leave at least 5 to 10 minutes between treatments. If treatment is combined with non-aqueous oily eye products, administer acetylcysteine eyedrops first.
Adverse reactions
Dogs and cats:
|
Very rare (<1 animal / 10,000 animals treated, including isolated reports): |
Application site reaction1 Eye irritation2, Eye inflammation2 (blinking, closed eyelid, eye redness, conjunctival oedema)3 |
1 mild and short, referring to discomfort in the eye occurring after the application of eye drops
2 and/or its adnexa
3 particularly in dogs.
As with any eye drops solution, mild and short discomfort reactions may occur upon administration.
Reporting adverse events is important. It allows continuous safety monitoring of a veterinary medicinal product. Reports should be sent, preferably via a veterinarian, to either the marketing authorisation holder or its local representative or the national competent authority via the national reporting system. See the package leaflet for respective contact details.
Dispensing
POM-V - Prescription Only Medicine – VeterinarianSUMMARY OF PRODUCT CHARACTERISTICS
1. NAME OF THE VETERINARY MEDICINAL PRODUCT
Stromease 25 mg/ml eye drops, solution for dogs and cats
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
Each ml contains:
Active substance:
|
Acetylcysteine Excipients: |
25.00 mg |
|
Benzalkonium chloride |
0.10 mg |
|
Dithiothreitol |
4.00 mg |
|
Disodium edetate |
0.50 mg |
For the full list of excipients, see section 6.1.
3. PHARMACEUTICAL FORM
Eye drops, solution
Clear colourless solution
4. CLINICAL PARTICULARS
4.1 Target species
Dogs and cats
4.2 Indications for use, specifying the target species
Supportive treatment of corneal ulcers.
4.3 Contraindications
Do not use in cases of known hypersensitivity to the active substance or any of the excipients.
4.4 Special warnings for each target species
None.
4.5 Special precautions for use
Special precautions for use in animals
Ocular re-examination should be made at frequent intervals during therapy. For the correct treatment of corneal ulceration, the underlying cause and/or the complicating factors should be identified and properly treated.
Special precautions to be taken by the person administering the veterinary medicinal product to animals
Wash hands after use.
4.6 Adverse reactions (frequency and seriousness)
As with any eye drops solution, mild and short discomfort reactions may occur upon administration.
Irritation or inflammation of the eye and/or its adnexa have been reported in very rare cases following use of the medicinal product.
In very rare cases, blinking of the eyelids or even transient closure of the eye, eye redness or conjunctival oedema have been reported, particularly in dogs, according to pharmacovigilance data.
The frequency of adverse reactions is defined using the following convention:
- very common (more than 1 in 10 animals treated displaying adverse reaction(s))
- common (more than 1 but less than 10 animals in 100 animals treated)
- uncommon (more than 1 but less than 10 animals in 1,000 animals treated)
- rare (more than 1 but less than 10 animals in 10,000 animals treated)
- very rare (less than 1 animal in 10,000 animals treated, including isolated reports).
4.7 Use during pregnancy, lactation or lay
Studies in rats and rabbits did not show toxicity in the pregnant female. The safety of the veterinary medicinal product has not been established during pregnancy and lactation in bitches or queens. Use only in accordance with the benefit-risk assessment by the responsible veterinarian.
4.8 Interaction with other medicinal products and other forms of interaction
None known.
4.9 Amounts to be administered and administration route
Ocular use.
The product is to be administered into the affected eye(s), at a dose of 2 eye drops, 3 to 4 times daily.
Instructions for opening the container and attachment of dropper applicator:
• Wash hands carefully in order to avoid microbiological contamination of the content in the vial.
• Flip open the metal cap and pull it all the way down along the pre-cut lines. Then remove the rest of the metal seal (picture 1).
• Remove the orange colored stopper (picture 2) from the vial.
• Do not touch the opening of the vial after removing the stopper.
• Take the dropper with the small white screw cap on the top out of its sachet, without touching the end intended to be attached to the vial, attach it (picture 3) to the vial and do not remove it anymore.
• The product is now ready for use (picture 4).
• 

Instructions for use:
Remove the small white screw cap to administer the product. Keep the dogs/cats head steady in a slightly upright position. Hold the container in an upright position without touching the eye. Rest your hand/little finger on the forehead of the dog/cat to maintain distance between the container and the eye. Gently pull the eyelid of the affected eye downwards, this will form a little eyelid pouch. Gently squeeze the dropper to administer two drops into the eyelid pouch that you created.
Be careful not to touch the dropper tip after opening the container and replace the white cap after use. Place the container back into the carton in the upright position and store out of sight and reach of children until the next medication.
Treatment should be continued in accordance with the instructions given by the individual veterinarian.
When treatment is combined with other ocular products, leave at least 5 to 10 minutes between treatments. If treatment is combined with non-aqueous oily eye products, administer acetylcysteine eyedrops first.
4.10 Overdose (symptoms, emergency procedures, antidotes), if necessary
Not known.
4.11 Withdrawal period(s)
Not applicable.
5. PHARMACOLOGICAL PROPERTIES
Pharmacotherapeutic group: Other ophthalmologicals, acetylcysteine ATC vet code: QS01XA08
5.1 Pharmacodynamic properties
Acetylcysteine is a mucolytic and proteolytic agent. N-acetylcysteine is a derivative of the amino acid l-cysteine and inhibits collagenase irreversibly by reducing disulphide bonds and chelating calcium and zinc. It also inhibits matrix metalloproteinase-9 (MMP-9) production by corneal epithelial cells.
Although MMPs play a role in initial corneal wound healing, down-regulation is necessary to prevent corneal digestion and allow corneal wound healing. The excipient dextran ensures good diffusion and prolonged contact time of the active ingredients.
5.2 Pharmacokinetic particulars
One study demonstrated, following application of radiolabelled cysteine, that acetylcysteine diffuses at the level of the cornea and the aqueous humor, resulting in intraocular penetration.
6. PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Disodium edetate
Benzalkonium chloride
Dithiothreitol
Dextran 70
Sodium dihydrogen phosphate dihydrate
Disodium phosphate
Sodium hydroxide (for pH adjustment)
Purified water
6.2 Major incompatibilities
Not applicable.
6.3 Shelf life
Shelf life of the veterinary medicinal product as packaged for sale: 3 years.
Shelf life after first opening the immediate packaging: 7 days
6.4. Special precautions for storage
This veterinary medicinal product does not require any special storage conditions.
6.5 Nature and composition of immediate packaging
Amber glass bottle type I containing 5 ml, with bromobutyl stopper type I and tear-off aluminium cap.
White PVC dropper with white HDPE cap.
Each vial is packed into a cardboard box.
6.6 Special precautions for the disposal of unused veterinary medicinal product or waste materials derived from the use of such products
Any unused veterinary medicinal product or waste materials derived from such veterinary medicinal product should be disposed of in accordance with local requirements.
7. MARKETING AUTHORISATION HOLDER
DOMES PHARMA
3 Rue André Citroën
63430 Pont-du-Château
FRANCE
8. MARKETING AUTHORISATION NUMBER(S)
Vm 54982/5008
9. DATE OF FIRST AUTHORISATION
26 October 2021
10 DATE OF REVISION OF THE TEXT
October 2024
![]()
Approved 07 October 2024
 TRUSTED SOURCE
TRUSTED SOURCE









