Other products
Sileo
Active substance
ATC code
Species
Dogs.
Indications
Alleviation of acute anxiety and fear associated with noise in dogs.
Dose to be administered and administration route
Oromucosal use.
The veterinary medicinal product should be administered onto the oral mucosa between dog’s cheek and gum at a dose of 125 micrograms/m2. The Sileo oral syringe is capable of delivering the veterinary medicinal product in 0.25 ml increments. Each increment is shown as one dot on the plunger. The dosing table provides the number of dots to be administered corresponding to the dog’s bodyweight.

The following dosing table provides the dose volume (in dots) to be administered for the corresponding bodyweight. If the dose for the dog is more than 6 dots (1.5 ml), half of the dose should be administered to the oral mucosa on one side of the dog’s mouth and the other half of the dose onto the other side. Do not exceed the recommended dose.
|
Bodyweight of dog (kg) |
Number of dots |
|
2.0–5.5 |
1 ● |
|
5.6–12 |
2 ●● |
|
12.1–20 |
3 ●●● |
|
20.1–29 |
4 ●●●● |
|
29.1–39 |
5 ●●●●● |
|
39.1–50 |
6 ●●●●●● |
|
50.1–62.5 |
7 ●●●●●●● |
|
62.6–75.5 |
8 ●●●●●●●● |
|
75.6–89 |
9 ●●●●●●●●● |
|
89.1–100 |
10 ●●●●●●●●●● |
The first dose should be given as soon as the dog shows the first signs of anxiety, or when the owner detects a typical stimulus (e.g. sound of fireworks or thunder) for eliciting anxiety or fear in the respective dog. Typical signs of anxiety and fear are panting, trembling, pacing (frequent change of place, running around, restlessness), seeking people (clinging, hiding behind, pawing, following), hiding (under furniture, in dark rooms), trying to escape, freezing (absence of movements), refusing to eat food or treats, inappropriate urination, inappropriate defecation, salivation, etc.
If the fear eliciting event continues and the dog shows signs of anxiety and fear again, re-dosing can be done when 2 hours have passed from the previous dose. The veterinary medicinal product can be dosed up to 5 times during each event.
Instructions for dosing the gel:
Dosing should be performed by an adult.
PREPARATIONS FOR DOSING:
 1. WEAR GLOVES
1. WEAR GLOVES
Wear impermeable disposable gloves when handling the veterinary medicinal product and handling the oral syringe.
 2. HOLD PLUNGER
2. HOLD PLUNGER
Hold the oral syringe plunger so that you can see the dot markings.
DOSE SELECTION AND DOSING:
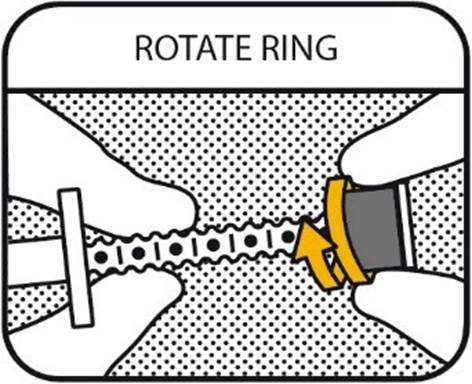 3. ROTATE RING
3. ROTATE RING
Hold the plunger and rotate the ring towards the barrel to select the dose your veterinarian has prescribed to your dog. Do not pull the plunger!
 4. SET THE DOSE
4. SET THE DOSE
Position the dosing ring so that the side nearest the barrel is in line with the graduation mark (black line), and the required number of dots shows between the dosing ring and the barrel.
 5. CONFIRM THE DOSE
5. CONFIRM THE DOSE
Make sure that you count the dots from the correct part of the plunger (shown in yellow) and that the ring is in line with the graduation mark (shown with the yellow arrow).
 6. FOLLOWING DOSES
6. FOLLOWING DOSES
To administer following doses from the same syringe: Repeat the previous “4. Set the dose” and “5. Confirm the dose” parts of the instructions.
 7. PULL CAP (TIGHT)
7. PULL CAP (TIGHT)
Pull the cap strongly while holding the barrel. Note the cap is very tight (pull, do not twist). Save the cap for replacement.
 8. DOSE INTO CHEEK
8. DOSE INTO CHEEK
Place the oral syringe tip between the dog’s cheek and gum and press the plunger until the dosing ring causes the plunger to stop.
 9. NOT SWALLOWED
9. NOT SWALLOWED
IMPORTANT: The gel should not be swallowed. If the gel is swallowed, it may not be effective.
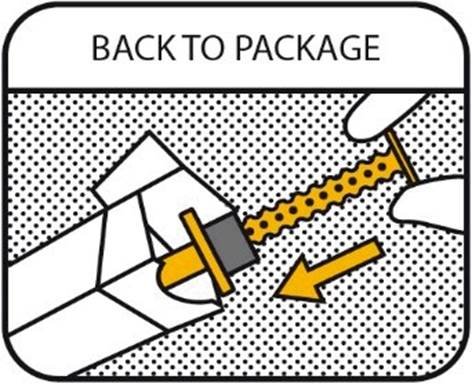 10. BACK TO PACKAGE
10. BACK TO PACKAGE
Recap the oral syringe and return it to the outer package as the veterinary medicinal product is sensitive to light. Make sure that the carton is closed properly. Keep the package out of sight and reach of children at all times. Remove and discard gloves.
Adverse reactions
Dogs:
| Common (1 to 10 animals / 100 animals treated): |
Emesis Sedation Urinary incontinence Pale mucous membrane1 |
| Uncommon (1 to 10 animals / 1 000 animals treated): |
Anxiety Gastroenteritis Periorbital oedema Drowsiness |
1Transient, observed at the application site due to peripheral vasoconstriction.
Reporting adverse events is important. It allows continuous safety monitoring of a veterinary medicinal product. Reports should be sent, preferably via a veterinarian, to either the marketing authorisation holder or its local representative or the national competent authority via the national reporting system. See the package leaflet for respective contact details.
Dispensing
POM-V - Prescription Only Medicine – VeterinarianReferences
1. NAME OF THE VETERINARY MEDICINAL PRODUCT
Sileo 0.1 mg/ml oromucosal gel for dogs
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
Each ml of the oromucosal gel contains:
Active substance:
Dexmedetomidine hydrochloride 0.1 mg (equivalent to 0.09 mg dexmedetomidine).
For the full list of excipients, see section 6.1.
3. PHARMACEUTICAL FORM
Oromucosal gel.
Translucent, green gel.
4. CLINICAL PARTICULARS
4.1 Target species
Dogs
4.2 Indications for use, specifying the target species
Alleviation of acute anxiety and fear associated with noise in dogs.
4.3 Contraindications
Do not use in dogs with severe cardiovascular disorders.
Do not use in dogs with severe systemic disease (graded as ASA III-IV) e.g. end stage renal or liver failure.
Do not use in known cases of hypersensitivity to the active substance or to any of the excipients.
Do not use in dogs obviously sedated from previous dosing.
4.4 Special warnings for each target species
None.
4.5 Special precautions for use
Special precautions for use in animals
If the oromucosal gel is swallowed it will become ineffective. Therefore feeding the dog or giving it treats within 15 minutes after administration of the gel should be avoided. In case the gel is swallowed the dog can be given another dose if necessary 2 hours after the previous dose.
In extremely nervous, excited or agitated animals, the levels of endogenous catecholamines are often high. The pharmacological response elicited by alpha-2 agonists (e.g. dexmedetomidine) in such animals may be reduced.
The safety of administering dexmedetomidine to puppies younger than 16 weeks and dogs over 17 years of age has not been studied.
Special precautions to be taken by the person administering the veterinary medicinal product to animals
In case of accidental ingestion or prolonged mucosal contact, seek medical advice immediately and show the package leaflet or the label to the physician. Do not drive as sedation and changes in blood pressure may occur.
Avoid skin, eye or mucosal contact. Wear impermeable disposable gloves when handling the veterinary medicinal product.
In case of skin contact wash the exposed skin immediately after exposure with large amounts of water and remove contaminated clothes. In case of eye or oromucosal contact, rinse abundantly with fresh water. If symptoms occur, seek the advice of a physician.
People with known hypersensitivity to dexmedetomidine or any of the excipients should avoid contact with the veterinary medicinal product.
Pregnant women should avoid contact with the product. Uterine contractions and decreased foetal blood pressure may occur after systemic exposure to dexmedetomidine.
Advice to the physician:
Dexmedetomidine, the active ingredient of Sileo is an alpha-2 adrenoceptor agonist. Symptoms after absorption may involve clinical effects including dose-dependent sedation, respiratory depression, bradycardia, hypotension, a dry mouth, and hyperglycaemia. Ventricular arrhythmias have also been reported. Since effects are dose dependent they are more pronounced in small children than adults. Respiratory and haemodynamic symptoms should be treated symptomatically. The specific alpha-2 adrenoceptor antagonist, atipamezole, which is approved for use in animals, has been used in humans but only experimentally to antagonize dexmedetomidine-induced effects.
4.6 Adverse reactions (frequency and seriousness)
Due to peripheral vasoconstriction, transient paleness of mucous membranes at the application site was commonly observed. Sedation, emesis and urinary incontinence were commonly observed in clinical trials.
Anxiety, periorbital oedema, drowsiness and signs of gastroenteritis were uncommonly observed in clinical trials.
The frequency of adverse reactions is defined using the following convention:
- very common (more than 1 in 10 animals treated displaying adverse reaction(s)
- common (more than 1 but less than 10 animals in 100 animals treated)
- uncommon (more than 1 but less than 10 animals in 1,000 animals treated)
- rare (more than 1 but less than 10 animals in 10,000 animals treated)
- very rare (less than 1 animal in 10,000 animals treated, including isolated reports).
4.7 Use during pregnancy, lactation or lay
The safety of this veterinary medicinal product has not been established during pregnancy and lactation in the target species.
Pregnancy and lactation
The use is not recommended during pregnancy and lactation.
4.8 Interaction with other medicinal products and other forms of interaction
The use of other central nervous system depressants is expected to potentiate the effects of dexmedetomidine and therefore an appropriate dose adjustment should be made.
4.9 Amounts to be administered and administration route
Oromucosal use.
The product should be administered onto the oral mucosa between dog’s cheek and gum at a dose of 125 micrograms/m2. The Sileo oral syringe is capable of delivering the product in 0.25 ml increments. Each increment is shown as one dot on the plunger. The dosing table provides the number of dots to be administered corresponding to the dog’s bodyweight.

The following dosing table provides the dose volume (in dots) to be administered for the corresponding bodyweight. If the dose for the dog is more than 6 dots (1.5 ml), half of the dose should be administered to the oral mucosa on one side of the dog’s mouth and the other half of the dose onto the other side. Do not exceed the recommended dose.
|
Bodyweight of dog (kg) |
Number of dots |
|
2.0–5.5 |
1 ● |
|
5.6–12 |
2 ●● |
|
12.1–20 |
3 ●●● |
|
20.1–29 |
4 ●●●● |
|
29.1–39 |
5 ●●●●● |
|
39.1–50 |
6 ●●●●●● |
|
50.1–62.5 |
7 ●●●●●●● |
|
62.6–75.5 |
8 ●●●●●●●● |
|
75.6–89 |
9 ●●●●●●●●● |
|
89.1–100 |
10 ●●●●●●●●●● |
The first dose should be given as soon as the dog shows the first signs of anxiety, or when the owner detects a typical stimulus (e.g. sound of fireworks or thunder) for eliciting anxiety or fear in the respective dog. Typical signs of anxiety and fear are panting, trembling, pacing (frequent change of place, running around, restlessness), seeking people (clinging, hiding behind, pawing, following), hiding (under furniture, in dark rooms), trying to escape, freezing (absence of movements), refusing to eat food or treats, inappropriate urination, inappropriate defecation, salivation, etc.
If the fear eliciting event continues and the dog shows signs of anxiety and fear again, re-dosing can be done when 2 hours have passed from the previous dose. The product can be dosed up to 5 times during each event.
Instructions for dosing the gel:
Dosing should be performed by an adult.
PREPARATIONS FOR DOSING:
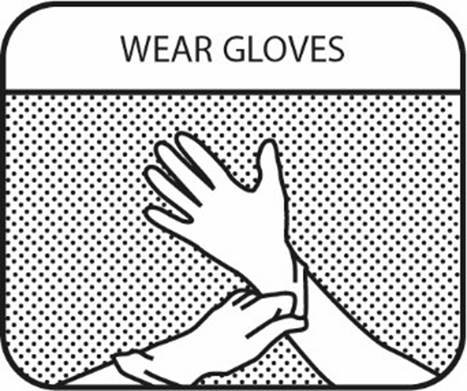 1. WEAR GLOVES
1. WEAR GLOVES
Wear impermeable disposable gloves when handling the veterinary medicinal product and handling the oral syringe.
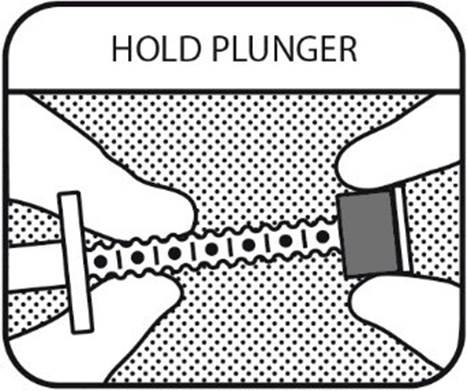 2. HOLD PLUNGER
2. HOLD PLUNGER
Hold the oral syringe plunger so that you can see the dot markings.
DOSE SELECTION AND DOSING:
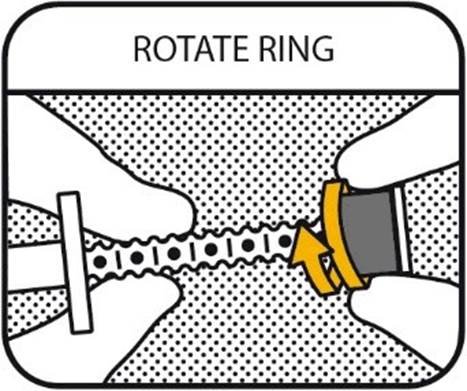 3. ROTATE RING
3. ROTATE RING
Hold the plunger and rotate the ring towards the barrel to select the dose your veterinarian has prescribed to your dog. Do not pull the plunger!
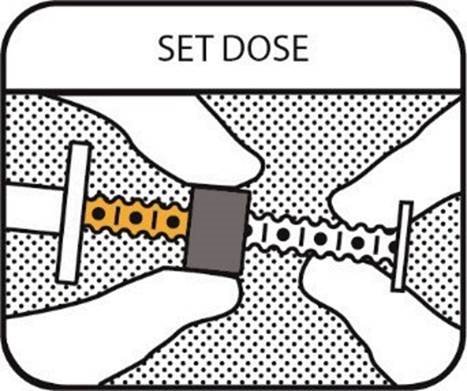 4. SET THE DOSE
4. SET THE DOSE
Position the dosing ring so that the side nearest the barrel is in line with the graduation mark (black line), and the required number of dots shows between the dosing ring and the barrel.
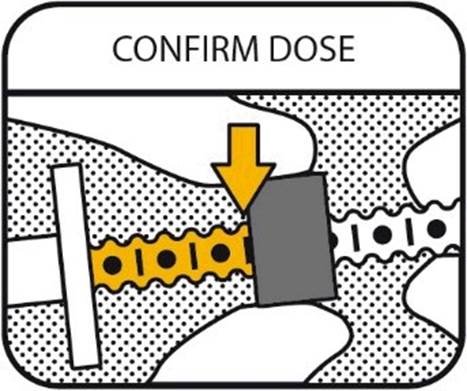 5. CONFIRM THE DOSE
5. CONFIRM THE DOSE
Make sure that you count the dots from the correct part of the plunger (shown in yellow) and that the ring is in line with the graduation mark (shown with the yellow arrow).
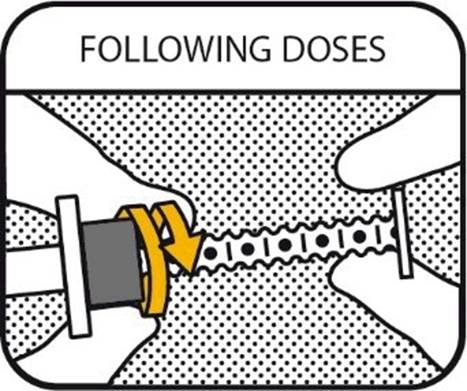 6. FOLLOWING DOSES
6. FOLLOWING DOSES
To administer following doses from the same syringe: Repeat the previous “4. Set the dose” and “5. Confirm the dose” parts of the instructions.
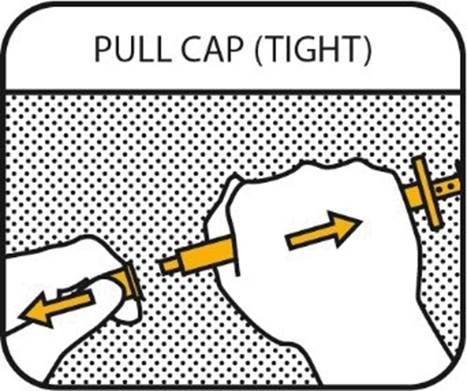 7. PULL CAP (TIGHT)
7. PULL CAP (TIGHT)
Pull the cap strongly while holding the barrel. Note the cap is very tight (pull, do not twist). Save the cap for replacement.
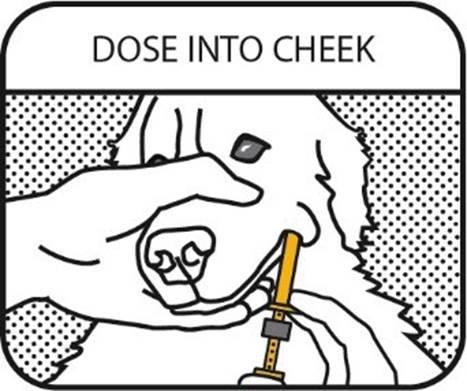 8. DOSE INTO CHEEK
8. DOSE INTO CHEEK
Place the oral syringe tip between the dog’s cheek and gum and press the plunger until the dosing ring causes the plunger to stop.
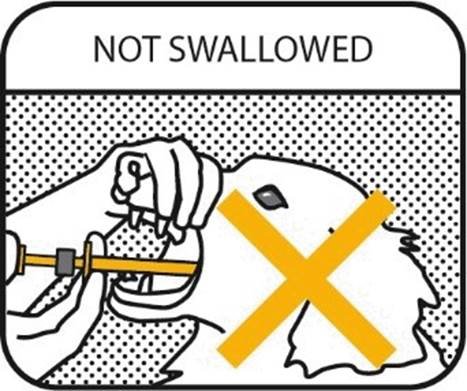 9. NOT SWALLOWED
9. NOT SWALLOWED
IMPORTANT: The gel should not be swallowed. If the gel is swallowed, it may not be effective.
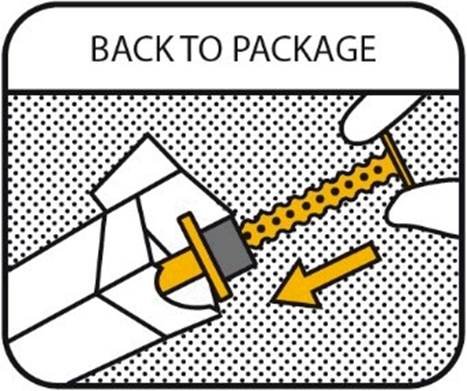 10. BACK TO PACKAGE
10. BACK TO PACKAGE
Recap the oral syringe and return it to the outer package as the product is sensitive to light. Make sure that the carton is closed properly. Keep the package out of sight and reach of children at all times. Remove and discard gloves.
4.10 Overdose (symptoms, emergency procedures, antidotes), if necessary
Signs of sedation may occur when the dose is exceeded. The level and duration of sedation is dose dependent. If sedation occurs, the dog should be kept warm.
Reduced heart rate may be seen after administration of higher than recommended doses of Sileo gel.
Blood pressure decreases slightly below normal levels. Respiration rate can occasionally decrease. Higher than recommended doses of Sileo gel may also induce a number of other alpha-2 adrenoceptor mediated effects, which include mydriasis, depression of motor and secretory functions of the gastrointestinal tract, temporary AV-blocks, diuresis and hyperglycaemia. A slight decrease in body temperature may be observed.
The effects of dexmedetomidine can be eliminated using a specific antidote, atipamezole (alpha-2 adrenoceptor antagonist). In case of overdose, the appropriate dose of atipamezole calculated in micrograms is 3 times (3X) the dose of administered dexmedetomidine hydrochloride in Sileo gel. Atipamezole (at the concentration of 5 mg/ml) dose in millilitres is one sixteenth (1/16th) of the dose volume of Sileo gel. 4.11 Withdrawal period(s) Not applicable.
5. PHARMACOLOGICAL PROPERTIES
Pharmacotherapeutic group: psycholeptics, hypnotics and sedatives.
ATCvet code: QN05CM18.
5.1 Pharmacodynamic properties
Sileo contains dexmedetomidine (as the hydrochloride salt) as the active substance. Dexmedetomidine is a potent and selective alpha-2 adrenoceptor agonist that inhibits the release of noradrenaline (NA) from noradrenergic neurons, blocks the startle reflex and thus counteracts arousal.
Dexmedetomidine as an alpha-2 adrenoceptor agonist alters the levels of NA, serotonin (5-HT) and dopamine (DA) in the hippocampus and frontal cortex, indicating that such compounds affect also the regions of the brain involved in creating and maintaining complex anxieties. In rodents alpha-2 adrenoceptor agonists reduce synthesis of NA, DA, 5-HT and the 5-HT precursor,
5-HTP (5-hydroxytryptophan), in the frontal cortex, hippocampus, striatum and hypothalamus and as a result decreases motor behaviour and signalling associated with distress.
In summary, dexmedetomidine, by decreasing central noradrenergic and serotonergic neurotransmission, is effective in alleviating canine acute anxiety and fear associated with noise. In addition to anxiolytic effect, dexmedetomidine has other well-known dose dependent pharmacological effects such as lowering of heart rate and rectal temperature, and peripheral vasoconstriction. These and other effects are described in more detail in section 4.10 on overdose.
5.2 Pharmacokinetic particulars
Oral bioavailability of dexmedetomidine is poor due to extensive first-pass metabolism. No measurable concentrations were found after gastro-intestinal gavage of dexmedetomidine to dogs. When administered via the oral mucosa, enhanced bioavailability is observed as a result of absorption in the oral cavity and the avoidance of first-pass metabolism in the liver.
The maximum concentration of dexmedetomidine occurs at about 0.6 hours after intramuscular or oromucosal administration. In a pharmacokinetic study in dogs the oromucosal mean bioavailability of dexmedetomidine was 28%. The apparent volume of distribution of dexmedetomidine in dogs is 0.9 l/kg. In the circulation, dexmedetomidine is largely bound to plasma proteins (93%). When studied in rats, the distribution of dexmedetomidine into rat tissues was rapid and wide with concentrations higher than in plasma for many tissues. Its levels in the brain were from 3-fold to 6-fold higher than the levels in plasma.
Dexmedetomidine is eliminated by biotransformation mainly in the liver, with a half-life in dogs ranging from 0.5 to 3 hours after oromucosal administration. Metabolism accounts for more than 98% of the elimination. Known metabolites show no or negligible activity. The major metabolic routes in dogs are hydroxylation of a methyl substituent and further oxidation to a carboxylic acid or Oglucuronidation of the hydroxylated product. N-methylation, N-glucuronidation and oxidation in the imidazole ring have also been observed. Metabolites are excreted mainly in the urine with a minor fraction found in the faeces.
6. PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Water, purified
Propylene glycol
Hydroxypropylcellulose
Sodium lauryl sulfate
Brilliant blue (E133)
Tartrazine (E102)
Sodium hydroxide (for pH-adjustment) Hydrochloric acid (for pH-adjustment)
6.2 Major incompatibilities Not applicable.
6.3 Shelf life
Shelf life of the veterinary medicinal product as packaged for sale: 3 years.
Shelf life after first opening the immediate packaging (removal of the cap): 4 weeks.
6.4. Special precautions for storage
Store the oral syringe in the carton in order to protect from light.
6.5 Nature and composition of immediate packaging
Pre-filled 3 ml HDPE oral syringes with graduations from 0.25 ml (1 dot) to 3 ml (12 dots). The oral syringe is fitted with a plunger, dosing ring and end cap (for sealing it).
Each oral syringe is packed in an individual child-resistant carton.
Pack sizes: single pack of 1 oral syringe and multipacks of 3 (3 packs of one), 5 (5 packs of one), 10 (10 packs of one) and 20 (20 packs of one).
Multipacks of 5, 10 and 20 oral syringes are intended to be supplied only to veterinarians.
Not all pack sizes may be marketed.
6.6 Special precautions for the disposal of unused veterinary medicinal product or waste materials derived from the use of such products
Any unused veterinary medicinal product or waste materials derived from such veterinary medicinal product should be disposed of in accordance with local requirements.
7. MARKETING AUTHORISATION HOLDER
Orion Corporation
Orionintie 1
FI-02200 Espoo
FINLAND
Tel.: +358 10 4261
8. MARKETING AUTHORISATION NUMBER(S)
EU/2/15/181/001–005
9. DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorisation: 10/06/2015 Date of last renewal: 24/04/2020
10. DATE OF REVISION OF THE TEXT
Detailed information on this veterinary medicinal product is available on the website of the European Medicines Agency (http://www.ema.europa.eu/).
PROHIBITION OF SALE, SUPPLY AND/OR USE
Not applicable.
 3. ROTATE RING
3. ROTATE RING 10. BACK TO PACKAGE
10. BACK TO PACKAGE TRUSTED SOURCE
TRUSTED SOURCE
 1. WEAR GLOVES
1. WEAR GLOVES 2. HOLD PLUNGER
2. HOLD PLUNGER 3. ROTATE RING
3. ROTATE RING 4. SET THE DOSE
4. SET THE DOSE 5. CONFIRM THE DOSE
5. CONFIRM THE DOSE 6. FOLLOWING DOSES
6. FOLLOWING DOSES 7. PULL CAP (TIGHT)
7. PULL CAP (TIGHT) 8. DOSE INTO CHEEK
8. DOSE INTO CHEEK 9. NOT SWALLOWED
9. NOT SWALLOWED 10. BACK TO PACKAGE
10. BACK TO PACKAGE