Other products
Tralieve vet. (20 kg)
Active substance
Species
Dogs.
Indications
For the reduction of acute and chronic mild soft tissue and musculoskeletal pain.
Dose to be administered and administration route
For oral use.
The recommended dose is 2-4 mg tramadol hydrochloride per kg body weight every 8 hours or as needed based on the intensity of pain.
Minimum dosing interval is 6 hours. The recommended maximum daily dose is 16 mg/kg. As the individual response to tramadol is variable and depends partly on the dosage, the age of the patient, individual differences in pain sensitivity and general condition, the optimal dosing regimen should be individually tailored using the above dose and re-treatment interval ranges. The dog should be examined regularly by a veterinarian to assess if additional analgesia is subsequently required. Additional analgesia can be administered by increasing the tramadol dose until the maximum daily dose is reached, and/or by following a multimodal analgesic approach with the addition of other suitable analgesics.
The most appropriate tablet strengths should be used in order to minimise divided tablets to be kept until the next dosing.
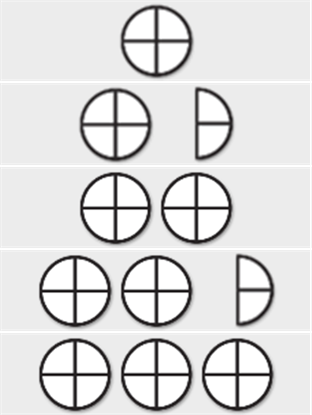
Please note that this dosing table is intended as a guide for dispensing the veterinary medicinal product at the high end of the dose range: 4 mg/kg bodyweight. It states the number of tablets required to administer 4 mg tramadol hydrochloride per kg bodyweight.
|
Body weight |
Tramadol 80 mg |
|
20 kg |
|
|
30 kg |
|
|
40 kg |
|
|
50 kg |
|
|
60 kg |
![]() = ¼ Tablet
= ¼ Tablet ![]() = ½ Tablet
= ½ Tablet ![]() = ¾ Tablet
= ¾ Tablet ![]() = 1 Tablet
= 1 Tablet
Tablets can be divided into 2 or 4 equal parts to ensure accurate dosing. Place the tablet on a flat surface, with its scored side facing up and the convex (rounded) side facing the surface.

2 equal parts: press down with your thumbs on both sides of the tablet. 4 equal parts: press down with your thumb in the middle of the tablet.
Adverse reactions
Dogs:
|
Common (1 to 10 animals / 100 animals treated): |
Sedationa,b, Drowsinessb |
|
Uncommon (1 to 10 animals / 1,000 animals treated): |
Nausea, Vomiting |
|
Rare (1 to 10 animals / 10,000 animals treated): |
Hypersensitivity reactionc |
|
Very rare (<1 animal / 10,000 animals treated, including isolated reports): |
Convulsiond |
a Mild.
b Especially when higher doses are given.
c The treatment should be discontinued.
d In dogs with a low seizures threshold.
Reporting adverse events is important. It allows continuous safety monitoring of a veterinary medicinal product. Reports should be sent, preferably via a veterinarian, to either the marketing authorisation holder or its local representative or the national competent authority via the national reporting system. See the package leaflet for respective contact details.
Dispensing
POM-V - Prescription Only Medicine – VeterinarianReferences
SUMMARY OF PRODUCT CHARACTERISTICS
1. NAME OF THE VETERINARY MEDICINAL PRODUCT
Tralieve 80 mg chewable tablets for dogs
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
80 mg tablet:
1 tablet contains:
Active substance:
Tramadol hydrochloride 80 mg
equivalent to 70.3 mg tramadol
Excipient(s):
For the full list of excipients, see section 6.1.
3. PHARMACEUTICAL FORM
Chewable tablet.
80 mg tablet: Light brown with brown spots, round and convex flavoured 11 mm tablet with a cross-shaped break line on one side.
Tablets can be divided into 2 or 4 equal parts.
4. CLINICAL PARTICULARS
4.1 Target species
Dogs.
4.2 Indications for use, specifying the target species
For the reduction of acute and chronic mild soft tissue and musculoskeletal pain.
4.3 Contraindications
Do not administer in conjunction with tricyclic antidepressants, monoamine oxidase inhibitors and serotonin reuptake inhibitors.
Do not use in cases of hypersensitivity to tramadol or to any of the excipients. Do not use in animals with epilepsy.
4.4 Special warnings
The analgesic effects of tramadol hydrochloride may be variable. This is thought to be due to individual differences in the metabolism of the drug to the primary active metabolite O-desmethyltramadol. In some dogs (non-responders) this may result in the product failing to provide analgesia. For chronic pain, multimodal analgesia should be considered. Dogs should be monitored regularly by a veterinarian to ensure adequate pain relief. In case of recurrence of pain or insufficient analgesia the analgesic protocol may need to be reconsidered.
4.5 Special precautions for use
Special precautions for use in animals
Use with caution in dogs with renal or hepatic impairment. In dogs with hepatic impairment the metabolism of tramadol to the active metabolites may be decreased which may reduce the efficacy of the product. One of the active metabolites of tramadol is renally excreted and therefore in dogs with renal impairment the dosing regimen used may need to be adjusted. Renal and hepatic function should be monitored when using this product. Cessation of long-term analgesic therapy should be done gradually whenever possible.
Special precautions to be taken by the person administering the veterinary medicinal product to animals
Tramadol may cause sedation, nausea and dizziness after accidental ingestion, especially by children. To avoid accidental ingestion, particularly by a child, unused tablet parts should be returned to the open blister space and inserted back into the carton and kept in a safe place out of the sight and reach of children as they pose a health risk to small children due to accidental ingestion. In case of accidental ingestion, particularly by children, seek medical advice and show the package leaflet or the label to the physician. In case of accidental ingestion by adults: DO NOT DRIVE as sedation may occur.
People with known hypersensitivity to tramadol or any of the excipients should avoid contact with the veterinary medicinal product. Wash hands after use.
4.6 Adverse reactions (frequency and seriousness)
Mild sedation and drowsiness may commonly occur, especially when higher doses are given.
Nausea and vomiting have uncommonly been observed in dogs after administration of tramadol.
In rare cases hypersensitivity can occur. In cases of hypersensitivity reactions the treatment should be discontinued.
In very rare cases tramadol may induce convulsions in dogs with a low seizure threshold.
The frequency of adverse reactions is defined using the following convention:
- very common (more than 1 in 10 animals treated displaying adverse reaction(s)
- common (more than 1 but less than 10 animals in 100 animals treated)
- uncommon (more than 1 but less than 10 animals in 1,000 animals treated)
- rare (more than 1 but less than 10 animals in 10,000 animals treated)
- very rare (less than 1 animal in 10,000 animals treated, including isolated reports)
4.7 Use during pregnancy and lactation
Pregnancy:
Laboratory studies in mice and/or rats and rabbits have not produced any evidence of teratogenic, foetotoxic, maternotoxic effects. Use only according to the benefit-risk assessment by the responsible veterinarian.
Lactation:
Laboratory studies in mice and/or rats and rabbits have not produced any evidence of adverse effects in the peri- and postnatal development of offspring. Use only according to the benefit-risk assessment by the responsible veterinarian.
Fertility:
In laboratory studies in mice and/or rats and rabbits, the use of tramadol at therapeutic doses did not adversely affect reproductive performance and fertility in males and females. Use only according to the benefit-risk assessment by the responsible veterinarian.
4.8 Interaction with other medicinal products and other forms of interaction
Concomitant administration of the product with central nervous system depressants, may potentiate the CNS and respiratory depressant effects.
Tramadol can increase the effect of drugs that lower the seizure threshold.
Drugs that inhibit (e.g. cimetidine and erythromycin) or induce (e.g. carbamazepine) CYP450 mediated metabolism may have an effect on the analgesic effect of tramadol. The clinical relevance of these interactions has not been studied in dogs. The combination with mixed agonist/antagonists (e.g. buprenorphine, butorphanol) and tramadol is not advisable, because the analgesic effect of a pure agonist may be theoretically reduced in such circumstances. See also section 4.3.
4.9 Amounts to be administered and administration route
For oral administration.
The recommended dose is 2-4 mg tramadol hydrochloride per kg body weight every 8 hours or as needed based on the intensity of pain.
Minimum dosing interval is 6 hours. The recommended maximum daily dose is 16 mg/kg. As the individual response to tramadol is variable and depends partly on the dosage, the age of the patient, individual differences in pain sensitivity and general condition, the optimal dosing regimen should be individually tailored using the above dose and re-treatment interval ranges. The dog should be examined regularly by a veterinarian to assess if additional analgesia is subsequently required. Additional analgesia can be administered by increasing the tramadol dose until the maximum daily dose is reached, and/or by following a multimodal analgesic approach with the addition of other suitable analgesics.
The most appropriate tablet strengths should be used in order to minimise divided tablets to be kept until the next dosing.
Please note that this dosing table is intended as a guide for dispensing the product at the high end of the dose range: 4 mg/kg bodyweight. It states the number of tablets required to administer 4 mg tramadol hydrochloride per kg bodyweight.
|
Body weight |
Tramadol 80 mg |
|
20 kg |
|
|
30 kg |
|
|
40 kg |
|
|
50 kg |
|
|
60 kg |
![]() = ¼ Tablet
= ¼ Tablet ![]() = ½ Tablet
= ½ Tablet ![]() = ¾ Tablet
= ¾ Tablet ![]() = 1 Tablet
= 1 Tablet
Tablets can be divided into 2 or 4 equal parts to ensure accurate dosing. Place the tablet on a flat surface, with its scored side facing up and the convex (rounded) side facing the surface.

2 equal parts: press down with your thumbs on both sides of the tablet. 4 equal parts: press down with your thumb in the middle of the tablet.
4.10 Overdose (symptoms, emergency procedures, antidotes)
In cases of intoxication with tramadol symptoms similar to those observed with other centrally acting analgesics (opioids) are likely to occur. These include in particular miosis, vomiting, cardiovascular collapse, consciousness disorders up to coma, convulsions and respiratory depression up to respiratory arrest.
General emergency measures: Maintain a patent airway, support cardiac and respiratory function depending on the symptoms. Inducing vomiting in order to empty the stomach is suitable unless the affected animal is showing reduced consciousness, in which case gastric lavage may be considered. The antidote for respiratory depression is naloxone. However, naloxone may not be useful in all cases of tramadol overdose as it may only partially reverse some of the other effects of tramadol. In case of seizures, administer diazepam.
4.11 Withdrawal period(s)
Not applicable
5. PHARMACOLOGICAL PROPERTIES
Pharmacotherapeutic group: Opioid analgesics, other opioids ATCvet Code: QN02AX02
5.1 Pharmacodynamic properties
Tramadol is a centrally acting analgesic agent with a complex mode of action exerted by its 2 enantiomers and primary metabolite, involving opioid, norepinephrine, and serotonin receptors. The (+) enantiomer of tramadol has a low affinity for the µ-opioid receptors, inhibits serotonin uptake and enhances its release. The (-) enantiomer preferentially inhibits norepinephrine reuptake. The metabolite O-desmethyltramadol (M1) has greater affinity for the µ-opioid receptors.
Unlike morphine, tramadol does not have depressing effects on respiration for an extensive analgesic dose range. Likewise, it does not affect gastrointestinal motility. The effects on the cardiovascular system tend to be mild. The analgesic potency of tramadol is about 1/10 to 1/6 of that of morphine.
5.2 Pharmacokinetic particulars
Tramadol is readily absorbed: After a single oral administration of 4.4 mg tramadol HCL per kg bodyweight, peak plasma concentrations of 65 ng tramadol per mL are achieved within 45 minutes. Food does not significantly affect the absorption of the drug.
Tramadol is metabolized in the liver by cytochrome P450 mediated demethylation followed by conjugation with glucuronic acid. In dogs, lower levels of the active metabolite O-desmethyltramadol are formed compared to humans. Elimination occurs mainly via the kidneys with an elimination half-life of about 0.5-2 hours.
6. PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Cellulose, microcrystalline
Lactose monohydrate
Sodium starch glycolate (type A)
Magnesium stearate
Silica, colloidal hydrated
Chicken flavour
Yeast (dried)
6.2 Major incompatibilities
Not applicable
6.3 Shelf life
Shelf life of the veterinary medicinal product as packaged for sale: 2 years.
Shelf life of divided tablets after first opening the immediate packaging: 3 days.
6.4 Special precautions for storage
Do not store above 30°C.
Store in the original package in order to protect from moisture.
6.5 Nature and composition of immediate packaging
Aluminium - PVC/PE/PVDC blister
Cardboard box of 1, 2, 3, 4, 5, 6, 7, 8, 9, 10 or 25 blisters of 10 tablets.
Cardboard box containing 10 separate cardboard boxes, each containing 3 blister of 10 tablets.
Not all pack sizes may be marketed.
6.6 Special precautions for the disposal of unused veterinary medicinal product or waste materials derived from the use of such products
Any unused veterinary medicinal product or waste materials derived from such veterinary medicinal products should be disposed of in accordance with local requirements.
7. MARKETING AUTHORISATION HOLDER
Dechra Regulatory B.V.
Handelsweg 25 5531 AE Bladel
The Netherlands
8. MARKETING AUTHORISATION NUMBER
Vm 50406/4019
9. DATE OF FIRST AUTHORISATION
09 July 2018
10. DATE OF REVISION OF THE TEXT
October 2022 
Approved: 27 October 2022

| Art. Nr. | 50406/5011 |
|---|---|
| EAN | 3858888795252 |

 TRUSTED SOURCE
TRUSTED SOURCE









