Combination therapy
No results were found for your selected species
Tessie
Active substance
ATC code
Species
Dogs
Indications
Short term alleviation of situational anxiety and fear in dogs triggered by noise or owner departure.
Dose to be administered and administration route
Oral use.
The product is intended for short term use but it can be safely administered for up to 9 consecutive days.
The product should be administered orally at a dose of 0.1 ml/kg bodyweight (equivalent to 30 mcg/kg) in situational anxiety and fear in dogs triggered by noise or owner departure.
If the product is intended for use in situations where the dog is meant to be alone following the administration, a test dose should be given. Following administration of the test dose the dog should be observed for 2 hours to make sure the selected dose of the product is not associated with adverse reactions and that it is safe for the treated dog to be left alone (see section 3.5).
Do not feed the dog for one hour before to one hour after treatment as absorption may be delayed. A small treat can be given to ensure that the dog swallows the solution. Water can be freely available.
Observe the dog. If the fear triggering event continues and the dog starts to show signs of anxiety and fear again, re-dosing can be done when at least 3 hours has passed from the previous dose. The product can be dosed up to 3 times within every 24 hours.
Dose reduction
If the dog appears drowsy, its movements are uncoordinated or it responds to its owner’s call abnormally slowly after receiving treatment, the dose could be too high. The subsequent dose should be reduced to 2/3 of the volume of the previous dose, corresponding to 20 mcg/kg bodyweight. Dose reduction should be implemented following veterinary advice only.
Anxiety and fear triggered by noise:
The first dose should be given one hour before expected start of an anxiety triggering stimulus, as soon as the dog shows the first signs of anxiety, or when the owner detects a typical stimulus for eliciting anxiety or fear in the respective dog.
Anxiety and fear triggered by owner departure:
The dose should be given one hour before expected owner departure.
Instructions for administration:
Adverse reactions
Dogs:
|
Very common (> 1 animal / 10 animals treated): |
Emesis Lethargy |
|
Common (1 to 10 animals / 100 animals treated): |
Behavioural disorder (Barking, Avoidance, Increased reactivity) Diarrhoea, Gastroenteritis, Nausea Hypersensitivity reaction Leucopenia Ataxia, Sedation, Somnolence, Disorientation Urinary incontinence Anorexia, Pale mucous membranes, Polydipsia |
|
Undetermined frequency (cannot be estimated for the available data): |
Decreased heart rate1, Low blood pressure1 Decreased body temperature1 |
1observed in non-anxious animals
Reporting adverse events is important. It allows continuous safety monitoring of a veterinary medicinal product. Reports should be sent, preferably via a veterinarian, to either the marketing authorisation holder or its local representative or the national competent authority via the national reporting system. See the package leaflet for respective contact details.
Dispensing
POM-V - Prescription Only Medicine – VeterinarianSUMMARY OF PRODUCT CHARACTERISTICS
1. NAME OF THE VETERINARY MEDICINAL PRODUCT
Tessie 0.3 mg/ml oral solution for dogs
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
Each ml contains:
Active substance:
Tasipimidine 0.3 mg
(equivalent to 0.427 mg tasipimidine sulfate)
Excipients:
Sodium benzoate (E211) 0.5 mg
Tartrazine (E102)
Brilliant blue (E133)
For the full list of excipients, see section 6.1.
3. PHARMACEUTICAL FORM
Oral solution.
Clear green solution.
4. CLINICAL PARTICULARS
4.1 Target species
Dogs
4.2 Indications for use, specifying the target species
Short term alleviation of situational anxiety and fear in dogs triggered by noise or owner departure.
4.3 Contraindications
Do not use in cases of hypersensitivity to the active substance or to any of the excipients.
Do not use in dogs with moderate or severe systemic disease (graded as ASA III or greater), e.g. moderate to severe renal, liver or cardiovascular disease. Do not use in dogs obviously sedated (shows signs of e.g. drowsiness, uncoordinated movements, decreased responsiveness) from previous dosing.
See section 4.7.
4.4 Special warnings for each target species
Typical signs of anxiety and fear are panting, trembling, pacing (frequent change of place, running around, restlessness), seeking people (clinging, hiding behind, pawing, following), hiding (under furniture, in dark rooms), trying to escape, freezing (absence of movements), refusing to eat food or treats, inappropriate urination, inappropriate defecation, salivation, etc. These signs may be alleviated but may not be completely eliminated.
In extremely nervous, excited or agitated animals, the levels of endogenous catecholamines are often high. The pharmacological effect induced by alpha-2 agonists in such animals may be reduced.
Consideration should be given to use of a behavioural modification programme, especially when dealing with a chronic condition such as separation anxiety.
4.5 Special precautions for use Special precautions for use in animals
See also section 4.9.
If the dog is sedated (shows signs of e.g. drowsiness, uncoordinated movements, decreased responsiveness), do not leave the dog alone and withhold food and water.
The safety of administering tasipimidine to dogs younger than 6 months and older than 14 years of age or weighing less than 3 kg has not been studied. Use only according to a benefit-risk assessment by the responsible veterinarian.
The accuracy of the syringe is demonstrated only for doses of 0.2 ml and higher. Dogs requiring doses lower than 0.2 ml can therefore not be treated.
As a decrease of body temperature can occur after the administration, the treated animal should be kept at a suitable ambient temperature.
Tasipimidine may indirectly induce an increase of glycaemia. In diabetic animals, use according to a benefit-risk assessment by the veterinarian.
In case of vomiting after intake of the oral solution, maintain the usual recommended interval between two administrations (at least 3 hours) before administering the product again.
Special precautions to be taken by the person administering the veterinary medicinal product to animals
Exposure to tasipimidine may cause adverse effects such as sedation, respiratory depression, bradycardia and hypotension.
Avoid oral ingestion and skin contact including hand-to-mouth contact.
In order to prevent children from getting access to the product, don’t leave the filled dosing syringe unattended while preparing the dog for administration. The used syringe and the closed bottle should be returned to the original carton and stored out of the sight and reach of children.
In case of skin contact, wash the exposed skin immediately with water and remove contaminated clothes. In case of accidental ingestion, seek medical advice immediately and show the package leaflet or the label to the physician. Do not drive, as sedation and changes in blood pressure may occur.
This product may cause slight eye irritation. Avoid eye contact including hand-to-eye contact. In case of eye contact, rinse the eyes immediately with water.
This veterinary medicinal product may cause hypersensitivity (allergy). People with known hypersensitivity to tasipimidine or any of the excipients should avoid contact with the veterinary medicinal product.
Wash hands after use.
4.6 Adverse reactions (frequency and seriousness)
Lethargy and emesis were very common adverse reactions in clinical trials.
Sedation, behavioural disorders (barking, avoidance, disorientation, increased reactivity), pale mucous membranes, ataxia, diarrhoea, urinary incontinence, nausea, gastroenteritis, polydipsia, leucopenia, hypersensitivity reactions, somnolence and anorexia were common adverse reactions in clinical trials.
Additionally, decrease in heart rate, blood pressure and body temperature were observed in preclinical studies in non-anxious animals.
The frequency of adverse reactions is defined using the following convention:
- very common (more than 1 in 10 animals treated displaying adverse reactions)
- common (more than 1 but less than 10 animals in 100 animals treated)
- uncommon (more than 1 but less than 10 animals in 1,000 animals treated)
- rare (more than 1 but less than 10 animals in 10,000 animals treated)
- very rare (less than 1 animal in 10,000 animals treated, including isolated reports).
4.7 Use during pregnancy, lactation or lay
Laboratory studies in rats have shown evidence of developmental toxicity at maternotoxic doses causing clear sedation-related clinical signs, decreased food consumption, and decreased body weight gain of the dam.
The safety of the veterinary medicinal product has not been established during pregnancy and lactation in the target species.
Do not use during pregnancy and lactation.
4.8 Interaction with other medicinal products and other forms of interaction
The safety of tasipimidine in combination with tricyclic antidepressant clomipramine (1.2-2.0 mg/kg), serotonin reuptake inhibitor fluoxetine (1.1-1.6 mg/kg), anaesthesia premedications (dexmedetomidine, methadone), induction agents (propofol) and inhalation anaesthetics (isoflurane) has been demonstrated in small-scale studies (N = 4-7) in laboratory dogs. When used concomitantly with clomipramine or fluoxetine, the tasipimidine dose should be reduced to 20 µg/kg bodyweight.
The use of other central nervous system depressants is expected to potentiate the effects of tasipimidine and therefore an appropriate dose adjustment should be made.
4.9 Amounts to be administered and administration route
Oral use.
The product is intended for short term use but it can be safely administered for up to 9 consecutive days.
The product should be administered orally at a dose of 0.1 ml/kg bodyweight (equivalent to 30 µg/kg) in situational anxiety and fear in dogs triggered by noise or owner departure.
If the product is intended for use in situations where the dog is meant to be alone following the administration, a test dose should be given. Following administration of the test dose the dog should be observed for 2 hours to make sure the selected dose of the product is not associated with adverse reactions and that it is safe for the treated dog to be left alone (see section 4.5).
Do not feed the dog for one hour before to one hour after treatment as absorption may be delayed. A small treat can be given to ensure that the dog swallows the solution. Water can be freely available.
Observe the dog. If the fear triggering event continues and the dog starts to show signs of anxiety and fear again, re-dosing can be done when at least 3 hours has passed from the previous dose. The product can be dosed up to 3 times within every 24 hours.
Dose reduction
If the dog appears drowsy, its movements are uncoordinated or it responds to its owner’s call abnormally slowly after receiving treatment, the dose could be too high. The subsequent dose should be reduced to 2/3 of the volume of the previous dose, corresponding to 20 µg/kg bodyweight. Dose reduction should be implemented following veterinary advice only. When used concomitantly with clomipramine or fluoxetine, tasipimidine dose should be reduced to 20 µg/kg bodyweight (see section 4.8).
Anxiety and fear triggered by noise:
The first dose should be given one hour before expected start of an anxiety triggering stimulus, as soon as the dog shows the first signs of anxiety, or when the owner detects a typical stimulus for eliciting anxiety or fear in the respective dog.
Anxiety and fear triggered by owner departure:
The dose should be given one hour before expected owner departure.
Instructions for administration:
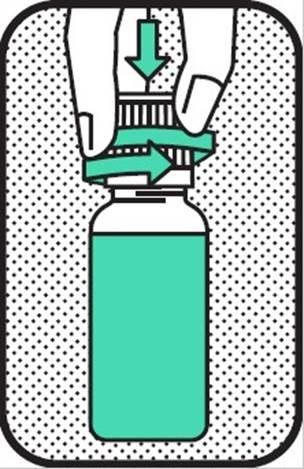 1. REMOVE CAP
1. REMOVE CAP
Remove the cap from the bottle (press down and twist). Save the cap for reclosure.

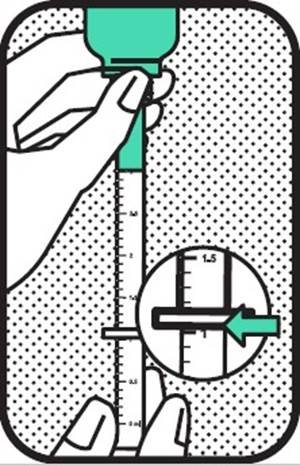 3. SELECT DOSE
3. SELECT DOSE
Turn the bottle with the syringe in place upside down. Pull the plunger out until the black line of correct dose (ml) can be seen under the grip plate of the syringe barrel.
If the dog weighs more than 30 kg, the total dose will be given in two separate doses as the syringe holds maximally 3.0 ml of solution.
The accuracy of the syringe is demonstrated only for doses of 0.2 ml and higher. Dogs requiring doses lower than 0.2 ml can therefore not be treated.
Don’t leave the filled dosing syringe unattended while preparing the dog for administration.
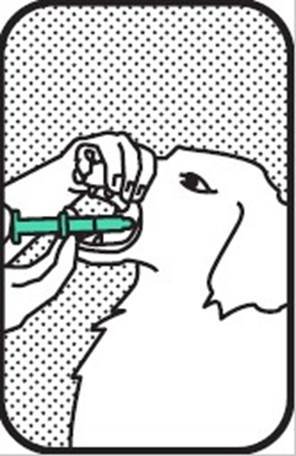 4. GIVE DOSE
4. GIVE DOSE
Gently place the syringe in the mouth of the dog and administer the dose to the base of the tongue by gradually pressing the plunger until the syringe is empty. Give the dog a small treat to ensure that the dog swallows the solution.
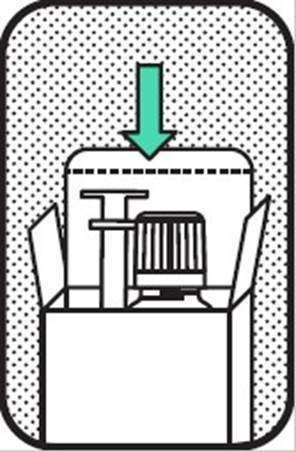 5. BACK TO PACKAGE
5. BACK TO PACKAGE
Replace the cap and rinse the syringe with water when finished. Put the syringe and bottle back to the secondary package and put them in the refrigerator.
4.10 Overdose (symptoms, emergency procedures, antidotes)
The level and duration of sedation is dose dependent, and signs of sedation may therefore particularly occur in case the dose is exceeded. Dogs receiving a high overdose of the product have a higher risk of aspirating vomit due to the emetic and CNS depressant effects associated with the active substance. A very high overdose can potentially be life-threatening.
Reduced heart rate may be seen after administration of higher than recommended doses of tasipimidine oral solution. Blood pressure decreases slightly below normal levels. Respiration rate can occasionally decrease. Higher than recommended doses of tasipimidine oral solution may also induce a number of other alpha-2 adrenoceptor mediated effects, which include increase in blood pressure, decrease in body temperature, lethargy, vomiting and a QT prolongation.
As demonstrated in a preclinical study, the effects of tasipimidine can be reversed using a specific antidote, atipamezole (alpha-2 adrenoceptor antagonist). One hour after treatment with tasipimidine at 60 µg/kg body weight, an atipamezole dose of 300 µg/kg bodyweight, corresponding to 0.06 ml/kg bodyweight of solution containing 5 mg/ml, was administered i.v. Results of this study demonstrated that the effects of tasipimidine could be reversed. However, as the half-life of tasipimidine exceeds that of atipamezole, some signs of tasipimidine effects may reappear.
4.11 Withdrawal period Not applicable.
5. PHARMACOLOGICAL PROPERTIES
Pharmacotherapeutic group: other hypnotics and sedatives
ATCvet code: QN05CM96
5.1 Pharmacodynamic properties
The veterinary medicinal product contains tasipimidine as the active substance. Tasipimidine is a potent and selective alpha-2A adrenoceptor agonist (as demonstrated in human adrenoceptors) that inhibits the release of noradrenaline from noradrenergic neurons, blocks the startle reflex and thus counteracts arousal.
Tasipimidine as an alpha-2 adrenoceptor agonist reduces the over activation of noradrenergic neurotransmission (increased release of noradrenaline in the locus coeruleus), which is shown to induce anxiety and fear in experimental animals exposed to stressful situations.
In summary, tasipimidine exerts its effects by decreasing central noradrenergic neurotransmission. In addition to the anxiolytic effect, tasipimidine can cause other known dose dependent alpha-2 adrenoceptor-mediated pharmacological effects such as sedation, analgesia and lowering of heart rate, blood pressure and rectal temperature.
The onset of effect is usually seen within 1 hour after treatment administration. The duration of effect shows some individual variation, and it can last for up to 3 hours or longer.
5.2 Pharmacokinetic particulars
Absorption
After oral administration in solution, tasipimidine is rapidly absorbed in fasted dogs. In a pharmacokinetic study in fasted dogs, a moderate oral bioavailability of tasipimidine was observed being on average 60%. After oral administration of 30 µg/kg to dogs in fasted state, the maximum plasma concentration of tasipimidine is approximately 5 ng/ml and occurs at 0.5–1.5 hours. When the dosing is repeated 3 hours later, the following maximum plasma concentration is shown to be moderately (30%) higher but there is no effect on the time of maximum concentration. Feeding at the time of dosing slows down the absorption and decreases the maximum plasma levels. In fed state the peak concentration is lower being 2.6 ng/ml and comes later at 0.7–6 hours.
The total plasma exposure to tasipimidine is comparable in fasted and fed states. Systemic exposure increases approximately in a dose proportional manner within the dose range of 10–100 µg/kg. No signs of accumulation are seen after repeated administration.
Distribution
Tasipimidine is a highly distributed compound, the volume of distribution in dogs is 3 l/kg. Tasipimidine penetrates the brain tissue in dogs and the drug concentration after repeated administration is higher in brain than in plasma. The in vitro binding of tasipimidine to dog plasma proteins is low, approximately 17%.
Metabolism
The metabolism of tasipimidine occurs mainly through demethylation and dehydrogenation and the most abundant circulating metabolites are demethylation and dehydrogenation products. The demethylated dehydrogenation product of tasipimidine is found in trace levels in dog plasma after high doses. The circulating metabolites are much less potent than the parent drug, as demonstrated in human and rat adrenoceptors.
Excretion
Tasipimidine is a highly cleared compound being rapidly eliminated from the circulation of dogs. The total clearance is 21 ml/min/kg after 10 µg/kg i.v. bolus dose. The mean terminal half-life is 1.7 hours after oral administration in fasted state. The portion of tasipimidine excreted unchanged in urine is 25%. All the circulating metabolites are excreted in urine much less compared to tasipimidine.
6. PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Sodium benzoate (E211)
Sodium citrate
Citric acid monohydrate
Brilliant blue (E133)
Tartrazine (E102)
Purified water
6.2 Major incompatibilities
In the absence of compatibility studies, this veterinary medicinal product must not be mixed with other veterinary medicinal products.
6.3 Shelf life
Shelf life of the veterinary medicinal product as packaged for sale: 3 years. Shelf life after first opening the immediate packaging: 12 months in a refrigerator (2°C to 8°C) or 1 month below 25°C.
6.4 Special precautions for storage
Store in a refrigerator (2°C to 8°C). Keep the bottle in the outer carton in order to protect from light.
6.5 Nature and composition of immediate packaging
15 ml clear glass type III bottle with a polypropylene child-resistant closure and a lowdensity polyethylene adapter and a high-density polyethylene liner. An oral lowdensity polyethylene/polystyrene syringe is included in the pack.
Pack sizes:
Cardboard box with 1 bottle and an oral syringe.
6.6 Special precautions for the disposal of unused veterinary medicinal product or waste materials derived from the use of such products
Any unused veterinary medicinal product or waste materials derived from such veterinary medicinal product should be disposed of in accordance with local requirements.
7. MARKETING AUTHORISATION HOLDER
Orion Corporation
Orionintie 1
FI-02200 Espoo
Finland
8. MARKETING AUTHORISATION NUMBER
Vm 06043/5004
9. DATE OF FIRST AUTHORISATION
08 August 2022
10. DATE OF REVISION OF THE TEXT
August 2023

Approved: 18 August 2023
 TRUSTED SOURCE
TRUSTED SOURCE
 1. REMOVE CAP
1. REMOVE CAP 3. SELECT DOSE
3. SELECT DOSE 4. GIVE DOSE
4. GIVE DOSE 5. BACK TO PACKAGE
5. BACK TO PACKAGE








